Details of the Drug
General Information of Drug (ID: DMIVZ3W)
| Drug Name |
ES-936
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
192820-78-3; ES 936; UNII-1XI90I177M; 5-METHOXY-1,2-DIMETHYL-3-(4-NITROPHENOXYMETHYL)INDOLE-4,7-DIONE; 5-methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione; 1XI90I177M; 5-methoxy-1,2-dimethyl-3-((4-nitrophenoxy)methyl)indole-4,7-dione; AC1NBNHW; CHEMBL357217; SCHEMBL3991028; CTK8E6509; DTXSID20172879; MolPort-006-168-362; ZINC587989; AKOS024457934; 1H-Indole-4,7-dione, 5-methoxy-1,2-dimethyl-3-((4-nitrophenoxy)methyl)-; API0010300; DB02400; ES936, > LS-191843; B5514; KS-00000189; J-012477
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
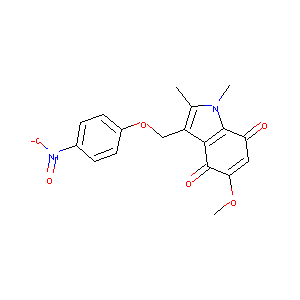 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 356.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
