Details of the Drug
General Information of Drug (ID: DMJBGI1)
| Drug Name |
CL-184005
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GF2IPH21J7; CL 184005; UNII-GF2IPH21J7; CL-184005; CHEMBL296973; 140466-18-8; CL-184,005; AC1L30Q7; DTXSID40161407; BDBM50001754; (2-methoxycarbonyl-3-tetradecoxyphenyl) [3-[(5-methyl-1,3-thiazol-3-ium-3-yl)methyl]phenyl] phosphate; ((CL 184005))3-{3-[Hydroxy-(2-methoxycarbonyl-3-tetradecyloxy-phenoxy)-phosphoryloxy]-benzyl}-5-methyl-thiazol-3-ium; 2-{Hydroxy-[3-(5-methyl-thiazol-3-ylmethyl)-phenoxy]-dihydrogen phosphate}-6-tetradecyloxy-benzoic acid methyl ester(CL 184005); Thiazolium, 3-((3-((hydroxy(2-(methoxycarbony
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
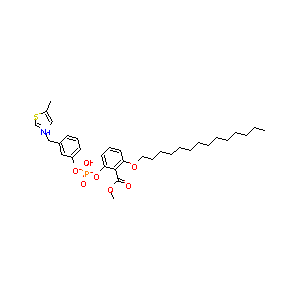 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 631.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 10.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 22 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Sepsis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1G40-1G41 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
