Details of the Drug
General Information of Drug (ID: DMJCF1O)
| Drug Name |
Maralixibat
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Lopixibat; UNII-UYB6UOF69L; Maralixibat [USAN]; 716313-53-0; UYB6UOF69L; CHEMBL363392; Maralixibat (USAN); Lopixibat cation; CHEMBL17879; 1-(4-((4-((4R,5R)-3,3-dibutyl-7-(dimethylamino)-4-hydroxy-1,1-dioxido-2,3,4,5-tetrahydrobenzo[b]thiepin-5-yl)phenoxy)methyl)benzyl)-1,4-diazabicyclo[2.2.2]octan-1-ium; LUM001 CATION; LUM-001 CATION; SCHEMBL10013954; BDBM50140282; 4-Aza-1-azoniabicyclo(2.2.2)octane, 1-((4-((4-((4R,5R)-3,3-dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl)phenoxy)methyl)phenyl)methyl)-; D10951; Q27291331; (4R,5R)-5-[4-[[4-(1-aza-4-azoniabicyclo[2.2.2]octan-4-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2H-1$l^{6}-benzothiepin-4-ol; 1-{4-[4-((4R,5R)-3,3-Dibutyl-7-dimethylamino-4-hydroxy-1,1-dioxo-2,3,4,5-tetrahydro-1H-1lambda*6*-benzo[b]thiepin-5-yl)-phenoxymethyl]-benzyl}-4-aza-1-azonia-bicyclo[2.2.2]octane; chloride
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
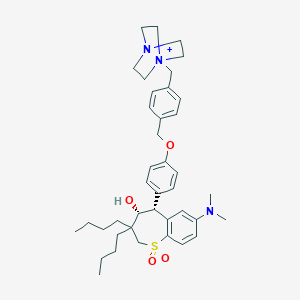 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 675 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.1 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pruritus | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EC90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
