| Synonyms |
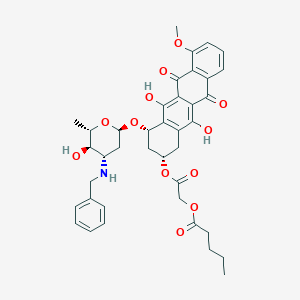
N-Benzyladriamycin-14-valerate; AD 198; 98983-21-2; Pentanoic acid, 2-(1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((phenylmethyl)amino)-alpha-L-lyxo-hexopyranoxyl)oxy)-2-naphthacenyl)-2-oxoethyl ester, (2S-cis)-; AC1L2RLY; AC1Q62MI; CTK8F7586; DTXSID40243893; 2-[(2s,4s)-4-{[3-(benzylamino)-2,3,6-trideoxy-; A-l-lyxo-hexopyranosyl]oxy}-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl]-2-oxoethyl pentanoate; LS-101847
|
| Chemical Identifiers |
- Formula
- C39H43NO12
- IUPAC Name
[2-[[(2S,4S)-4-[(2R,4S,5R,6S)-4-(benzylamino)-5-hydroxy-6-methyloxan-2-yl]oxy-5,12-dihydroxy-7-methoxy-6,11-dioxo-1,2,3,4-tetrahydrotetracen-2-yl]oxy]-2-oxoethyl] pentanoate - Canonical SMILES
-
CCCCC(=O)OCC(=O)O[C@@H]1C[C@@H](C2=C(C1)C(=C3C(=C2O)C(=O)C4=C(C3=O)C=CC=C4OC)O)O[C@H]5C[C@@H]([C@H]([C@@H](O5)C)O)NCC6=CC=CC=C6
- InChI
-
InChI=1S/C39H43NO12/c1-4-5-14-28(41)49-19-29(42)51-22-15-24-32(39(47)34-33(37(24)45)36(44)23-12-9-13-26(48-3)31(23)38(34)46)27(16-22)52-30-17-25(35(43)20(2)50-30)40-18-21-10-7-6-8-11-21/h6-13,20,22,25,27,30,35,40,43,45,47H,4-5,14-19H2,1-3H3/t20-,22-,25-,27-,30-,35-/m0/s1
- InChIKey
-
DFEZNIBKLJWMIQ-VEUGUUDJSA-N
|