Details of the Drug
General Information of Drug (ID: DMJGYPK)
| Drug Name |
AZD9056
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AZD-9056; AZD9056; 345304-65-6; UNII-F13K378W4L; N-(1-adamantylmethyl)-2-chloro-5-[3-(3-hydroxypropylamino)propyl]benzamide; F13K378W4L; AZD 9056; GTPL7826; SCHEMBL4126642; CHEMBL3545108; HSQAARMBHJCUOK-UHFFFAOYSA-N; MolPort-044-723-510; KS-000000WO; BCP25185; ZINC34356159; AKOS030228502; DB12594; Benzamide, 2-chloro-5-(3-((3-hydroxypropyl)amino)propyl)-N-(tricyclo(3.3.1.13,7)dec-1-ylmethyl)-; 2-Chloro-5-[3-[(3-hydroxypropyl)amino]propyl]-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)-benzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
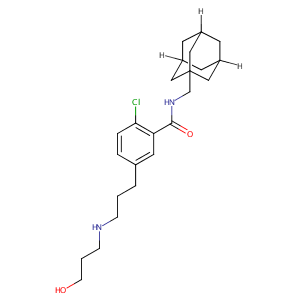 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 419 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic obstructive pulmonary disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA22 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
