Details of the Drug
General Information of Drug (ID: DMJHXMY)
| Drug Name |
1-benzyl-APDC
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL60238; 171336-76-8; (2R,4R)-1-BENZYL-4-AMINO-PYRROLIDINE-2,4-DIcarboxylic acid; GTPL1436; SCHEMBL8061968; CTK0A8043; DTXSID60573467; ZINC39952128; BDBM50071161; DB-064818; AJ-101168; FT-0772028; 4-amino-1-(phenylmethyl)pyrrolidine-2,4-dicarboxylic acid; (2R,4R)-4-amino-1-benzylpyrrolidine-2,4-dicarboxylic acid; (2R,4R)-4-Amino-1-benzyl-pyrrolidine-2,4-dicarboxylic acid; (2R)-1-Benzyl-4-aminopyrrolidine-2beta,4alpha-dicarboxylic acid; 4-Amino-1-benzyl-pyrrolidine-2,4-dicarboxylic acid(1-benzyl-APDC)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
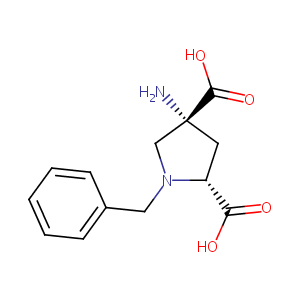 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 264.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
