Details of the Drug
General Information of Drug (ID: DMJKAQR)
| Drug Name |
L-790070
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
L-790070; CHEMBL303144; 189442-43-1; MRK-48; SCHEMBL4025110; BDBM15459; CTK4E0131; DTXSID50430959; 4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluoromethyl)phenyl]-1H-imidazol-5-yl]-N-[(1S)-1-phenylethyl]pyridin-2-amine; 4-{1-methyl-2-piperidin-4-yl-4-[3-(trifluoromethyl)phenyl]-1H-imidazol-5-yl}-N-[(1S)-1-phenylethyl]pyridin-2-amine; 2-PYRIDINAMINE, 4-[1-METHYL-2-(4-PIPERIDINYL)-4-[3-(TRIFLUOROMETHYL)PHENYL]-1H-IMIDAZOL-5-YL]-N-[(1S)-1-PHENYLETHYL]-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
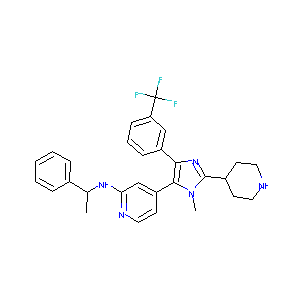 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 505.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Autoimmune diabetes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
