Details of the Drug
General Information of Drug (ID: DMJQVAG)
| Drug Name |
S-ATPO
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL594840; (S)-2-Amino-3-(5-Tert-Butyl-3-(Phosphonomethoxy)-4-Isoxazolyl)propionic Acid; 2-AMINO-3-(5-TERT-BUTYL-3-(PHOSPHONOMETHOXY)-4-ISOXAZOLYL)PROPIONIC ACID; (2S)-2-amino-3-[5-tert-butyl-3-(phosphonomethoxy)-1,2-oxazol-4-yl]propanoic acid; AT1; 1n0t; AC1L9KN0; (S)-ATPO; SCHEMBL5457168; BCP16890; ZINC2047684; BDBM50304093; AKOS032960456; DB02347; A-330; 3-[5-tert-butyl-3-(phosphonomethoxy)isoxazol-4-yl]-L-alanine; (S)-2-amino-3-[5-tertbutyl-3-(phosphono-methoxy)-4-isoxazolyl]-propionic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
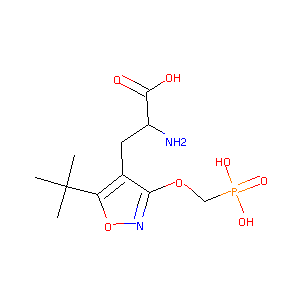 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 322.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
