Details of the Drug
General Information of Drug (ID: DMJRD7A)
| Drug Name |
GSK3326595
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
JLCCNYVTIWRPIZ-NRFANRHFSA-N; 1616392-22-3; 6-[(1-acetylpiperidin-4-yl)amino]-N-[(2S)-2-hydroxy-3-(1,2,3,4-tetrahydroisoquinolin-2-yl)propyl]pyrimidine-4-carboxamide; SCHEMBL15825290; MolPort-044-813-754; BDBM178166; AKOS030528008; ZINC220119494; CS-6451; AS-53150; HY-101563; GSK3326595(EPZ015938); US9675614, 208; (S)-6-((1-acetylpiperidin-4-yl)amino)-N-(3-(3,4-dihydroisoquinolin-2(1H)-yl)-2-hydroxypropyl)pyrimidine-4-carboxamide
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
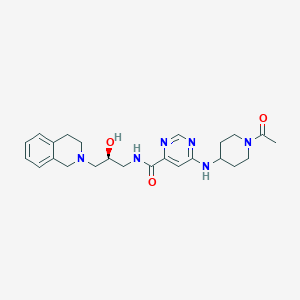 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 452.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.1 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
