| Drug Name |
NB-598
|
| Synonyms |
KIRGLCXNEVICOG-SOFGYWHQSA-N; 131060-14-5; NB 598; CHEMBL27885; (E)-3-[(3,3'-Bithiophen)-5-ylmethoxy]-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-ethylbenzylamine; (2E)-N-({3-[([3,3'-bithiophen]-5-yl)methoxy]phenyl}methyl)-N-ethyl-6,6-dimethylhept-2-en-4-yn-1-amine; AC1O5YMV; SCHEMBL9454494; C27H31NOS2; GTPL3103; SCHEMBL9454490; (E)-N-ethyl-6,6-dimethyl-N-[[3-[(4-thiophen-3-ylthiophen-2-yl)methoxy]phenyl]methyl]hept-2-en-4-yn-1-amine; ZINC2003883; BCP28146; EX-A1748; BDBM50032850; 3914AH; AKOS030526399; CS-1274; NCGC00165845-01
|
| Drug Type |
Small molecular drug
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
449.7 |
|
| Logarithm of the Partition Coefficient (xlogp) |
7.2 |
| Rotatable Bond Count (rotbonds) |
10 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| Chemical Identifiers |
- Formula
- C27H31NOS2
- IUPAC Name
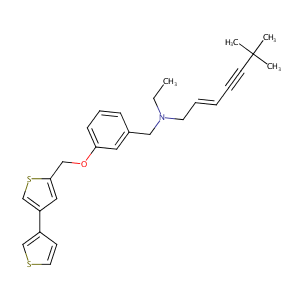
(E)-N-ethyl-6,6-dimethyl-N-[[3-[(4-thiophen-3-ylthiophen-2-yl)methoxy]phenyl]methyl]hept-2-en-4-yn-1-amine - Canonical SMILES
-
CCN(C/C=C/C#CC(C)(C)C)CC1=CC(=CC=C1)OCC2=CC(=CS2)C3=CSC=C3
- InChI
-
InChI=1S/C27H31NOS2/c1-5-28(14-8-6-7-13-27(2,3)4)18-22-10-9-11-25(16-22)29-19-26-17-24(21-31-26)23-12-15-30-20-23/h6,8-12,15-17,20-21H,5,14,18-19H2,1-4H3/b8-6+
- InChIKey
-
KIRGLCXNEVICOG-SOFGYWHQSA-N
|
| Cross-matching ID |
- PubChem CID
- 6443223
- CAS Number
-
- TTD ID
- D0I3XW
|
|
|
|
|
|
|
|


