Details of the Drug
General Information of Drug (ID: DMJVZBC)
| Drug Name |
TP-0184
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Itacnosertib; UNII-22Z53X5LHF; 22Z53X5LHF; 1628870-27-8; methylpiperazin-1-yl)phenyl]pyrimidine-2,4-diamine; N4-([2,2'-bipyridin]-3-yl)-N2-[3-methoxy-4-(4-; 2-N-[3-methoxy-4-(4-methylpiperazin-1-yl)phenyl]-4-N-(2-pyridin-2-ylpyridin-3-yl)pyrimidine-2,4-diamine; Itacnosertib [INN]; SCHEMBL16129103; GTPL10632; TP0184; example 11 [WO2014151871A9]; 2,4-Pyrimidinediamine, N4-(2,2'-bipyridin)-3-yl-N2-(3-methoxy-4-(4-methyl-1-piperazinyl)phenyl)-; N2-(3-Methoxy-4-(4-methylpiperazin-1-yl)phenyl)-N4-(2-(pyridin-2-yl)pyridin-3-yl)pyrimidine-2,4-diamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
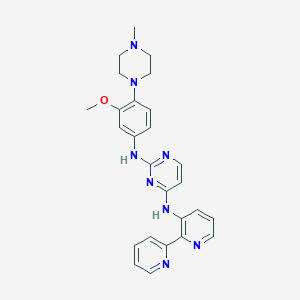 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 468.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
