Details of the Drug
General Information of Drug (ID: DMK7BND)
| Drug Name |
Briciclib
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Briciclib; 865783-99-9; ON-014185; UNII-WG93X96336; WG93X96336; ON 014185; Briciclib [USAN:INN]; Briciclib (USAN/INN); Briciclib; ON 014185; SCHEMBL1634579; SCHEMBL1634581; CHEMBL1206245; MolPort-046-033-539; LXENKEWVEVKKGV-BQYQJAHWSA-N; EX-A2492; BCP17292; ZINC28965775; AKOS027439966; DB12004; CS-5589; HY-16366; KB-79924; Briciclib(ON 013105 ON 014185); ON-013105; D10614; (2-methoxy-5-{[(E)-2-(2,4,6-trimethoxyphenyl)ethenesulfonyl]methyl}phenoxy)phosphonic acid; (e)-5-((2,4,6-trimethoxystyrylsulfonyl)methyl)-2-methoxyphenyl dihydro
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
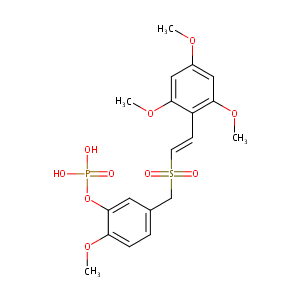 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 474.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
