Details of the Drug
General Information of Drug (ID: DMK9IPF)
| Drug Name |
JNJ-73841937
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Lazertinib; 1903008-80-9; GNS-1480; YH25448; YH-25448; UNII-4A2Y23XK11; GNS1480; 4A2Y23XK11; N-(5-((4-(4-((Dimethylamino)methyl)-3-phenyl-1H-pyrazol-1-yl)pyrimidin-2-yl)amino)-4-methoxy-2-morpholinophenyl)acrylamide; N-[5-[[4-[4-[(dimethylamino)methyl]-3-phenylpyrazol-1-yl]pyrimidin-2-yl]amino]-4-methoxy-2-morpholin-4-ylphenyl]prop-2-enamide; N-{5-[(4-{4-[(dimethylamino)methyl]-3-phenyl-1H-pyrazol-1-yl}pyrimidin-2-yl)amino]-4-methoxy-2-(morpholin-4-yl)phenyl}prop-2-enamide; Lazertinib [USAN]; Lazertinib [INN]; Lazertinib (YH25448,GNS-1480); CHEMBL4558324; SCHEMBL17670400; GTPL10136; BCP30440; EX-A1912; JNJ-73841937-AAA; s8724; WHO 10587; CCG-270023; YH-25448;GNS-1480; BS-15742; Compound 73 [WO2016060443A2]; HY-109061; AK00779508; CS-0032992; A16827; C-18112003-G; YH-25448; YH 25448; YH25448; GNS-1480; GNS 1480; GNS1480; 2-Propenamide, N-(5-((4-(4-((dimethylamino)methyl)-3-phenyl-1H-pyrazol-1-yl)-2-pyrimidinyl)amino)-4-methoxy-2-(4-morpholinyl)phenyl)-; CN(C)CC=1C(=NN(C=1)C1=NC(=NC=C1)NC=1C(=CC(=C(C=1)NC(C=C)=O)N1CCOCC1)OC)C1=CC=CC=C1; N-(5-((4-(4-((Dimethylamino)methyl)-3-phenyl-1H-pyrazol-1-yl)-2-pyrimidinyl)amino)-4-methoxy-2-(4-morpholinyl)phenyl)acrylamide; N-(5-(4-(4-((dimethylamino)methyl)-3-phenyl-1H-pyrazol-1-yl)pyrimidin-2-ylamino)-4-methoxy-2-morpholinophenyl)acrylamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
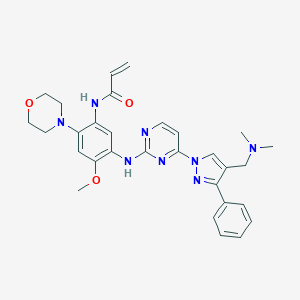 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
