Details of the Drug
General Information of Drug (ID: DMKDBFL)
| Drug Name |
HBR-985
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Glicondamide; 52994-25-9; UNII-876SH4764F; C18H20ClN3O5S; 876SH4764F; 1-[[p-[2-(5-chloro-o-anisamido)ethyl]phenyl]sulfonyl]-3-methylurea; Glicondamide [INN]; 1-((p-(2-(5-Chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-methylurea; AC1Q3LT7; CHEMBL12806; AC1L562G; SCHEMBL1818252; CTK4J6836; DTXSID10201026; ZINC537790; HB-985; AKOS030573774; ACM52994259; W0037; 5-chloro-2-methoxy-N-[2-[4-(methylcarbamoylsulfamoyl)phenyl]ethyl]benzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
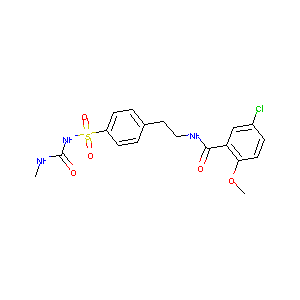 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 425.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
