| Drug Name |
US8933228, Ref 2
|
| Synonyms |
SCHEMBL2531614; CHEMBL3695569; AVUUHIYJPTWYNX-UHFFFAOYSA-N; BDBM142598; US8933228, Ref 2; N-(4-(4-(3-(3-tert-Butyl-1-p-tolyl-1H-pyrazol-5-yl)ureido)naphthalen-1-yloxy)pyridin-2-yl)-2-methoxyacetamide |
| Drug Type |
Small molecular drug
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 2 |
Molecular Weight (mw) |
578.7 |
|
| Logarithm of the Partition Coefficient (xlogp) |
6 |
| Rotatable Bond Count (rotbonds) |
9 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
6 |
| Chemical Identifiers |
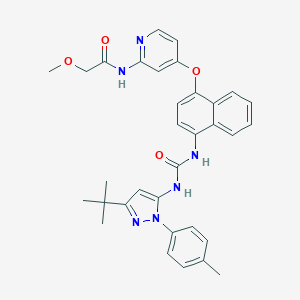
- Formula
- C33H34N6O4
- IUPAC Name
N-[4-[4-[[5-tert-butyl-2-(4-methylphenyl)pyrazol-3-yl]carbamoylamino]naphthalen-1-yl]oxypyridin-2-yl]-2-methoxyacetamide - Canonical SMILES
-
CC1=CC=C(C=C1)N2C(=CC(=N2)C(C)(C)C)NC(=O)NC3=CC=C(C4=CC=CC=C43)OC5=CC(=NC=C5)NC(=O)COC
- InChI
-
InChI=1S/C33H34N6O4/c1-21-10-12-22(13-11-21)39-30(19-28(38-39)33(2,3)4)37-32(41)35-26-14-15-27(25-9-7-6-8-24(25)26)43-23-16-17-34-29(18-23)36-31(40)20-42-5/h6-19H,20H2,1-5H3,(H,34,36,40)(H2,35,37,41)
- InChIKey
-
AVUUHIYJPTWYNX-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 46218331
- TTD ID
- D0Y4TK
|
|
|
|
|
|
|
|

