Details of the Drug
General Information of Drug (ID: DMKM3SX)
| Drug Name |
Sulfamerazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cremomerazine; Kelamerazine; Mebacid; Mesulfa; Methylpyrimal; Methylsulfadiazine; Methylsulfazin; Methylsulfazine;Metilsulfadiazin; Metilsulfazin; Percoccide; Pyralcid; Romezin; Septacil; Septosyl; Solfamerazina; Solumedin; Sulfameradine; Sulfamerazin; Sulfamerazina; Sulfamerazinum; Sulfamethyldiazine; Sulphamerazine; Sumedine; Pyrimal M; Solfamerazina [DCIT]; RP 2632; A-310; Debenal-M; Pirimal-M; Sulfamerazina [INN-Spanish]; Sulfamerazine (INN); Sulfamerazinum [INN-Latin]; Veta-Merazine; Sulfamerazine [USAN:INN:BAN]; N-(4-Methyl-2-pyrimidyl)sulfanilamide; N1-(4-Methyl-2-pyrimidinyl)sulfanilamide; N1-(4-Methylpyrimidin-2-yl)sulfanilamide; N(1)-(4-Methyl-2-pyrimidinyl)sulfanilamide; N(sup 1)-(4-Methyl-2-pyrimidinyl)sulfanilamide; N(sup1)-(4-Methyl-2-pyrimidinyl)sulfanilamide; Sulfanilamide, N1-(4-methyl-2-pyrimidinyl)-(8CI); (p-Aminobenzolsulfonyl)-2-amino-4-methylpyrimidin; (p-Aminobenzolsulfonyl)-2-amino-4-methylpyrimidin [German]; 2(p-Aminobenzolsulfonamido)-4-methylpyrimidin; 2-(4-Aminobenzenesulfonamido)-4-methylpyrimidine; 2-(Sulfanilamido)-4-methylpyrimidine; 2-(p-Aminobenzolsulfonamido)-4-methylpyrimidine; 2-Sulfa-4-methylpyrimidine; 2-Sulfanilamido-4-methylpyrimidine; 2643-RP; 4-Amino-N-(4-methyl-2-pyrimidinyl)-benzenesulfonamide; 4-Amino-N-(4-methyl-2-pyrimidinyl)-benzenesulfonamide (9CI); 4-Amino-N-(4-methyl-2-pyrimidinyl)benzenesulfonamide; 4-Amino-N-(4-methyl-pyrimidin-2-yl)-benzenesulfonamide; 4-amino-N-(4-methylpyrimidin-2-yl)benzene-1-sulfonamide; 4-amino-N-(4-methylpyrimidin-2-yl)benzenesulfonamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
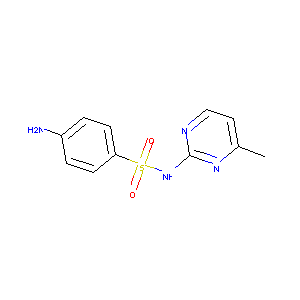 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 264.31 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
