Details of the Drug
General Information of Drug (ID: DMKNQW4)
| Drug Name |
7,8-dichloroquinoline-4-one-3-carboxylic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
300675-28-9; 7,8-DICHLORO-4-HYDROXYQUINOLINE-3-CARBOXYLIC ACID; 144061-33-6; 7,8-dichloro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 7,8-dichloro-4-oxo-1H-quinoline-3-carboxylic Acid; CHEMBL218566; 7,8-Dichloro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid; J-650168; 7,8-dichloro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; AC1LEVOV; Oprea1_635608; Oprea1_583373; SCHEMBL9654331; SCHEMBL17502115; CTK7I6370; CHEBI:94582; DTXSID00351180; MolPort-000-680-344; MolPort-000-651-074; ZINC3945161; STK539875; MFCD01909987
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
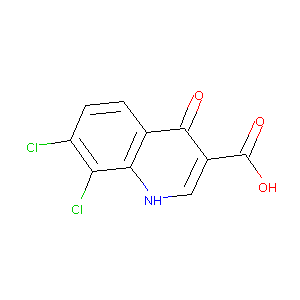 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 258.05 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
