| Synonyms |
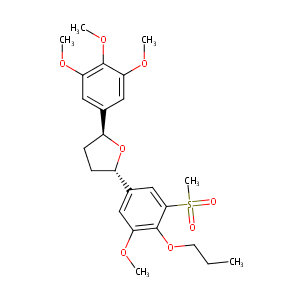
L-659989; 113787-28-3; L-659,989; L659989; L 659989; CHEMBL37781; SCHEMBL6154076; GTPL3426; DTXSID70433028; BDBM50002829; ZINC13650944; FT-0670693; L-662,418; L-659,989, (+); (2S,5S)-2-(3-methoxy-5-methylsulfonyl-4-propoxyphenyl)-5-(3,4,5-trimethoxyphenyl)oxolane; (2R,5R)-rel-Tetrahydro-2-[3-methoxy-5-(methylsulfonyl)-4-propoxyphenyl]-5-(3,4,5-trimethoxyphenyl)furan; trans-( inverted exclamation markA)-Tetrahydro-2-[3-methoxy-5-(methylsulfonyl)-4-propoxyphenyl]-5-(3,4,5-trimethoxyphenyl)furan
|
| Chemical Identifiers |
- Formula
- C24H32O8S
- IUPAC Name
(2S,5S)-2-(3-methoxy-5-methylsulfonyl-4-propoxyphenyl)-5-(3,4,5-trimethoxyphenyl)oxolane - Canonical SMILES
-
CCCOC1=C(C=C(C=C1S(=O)(=O)C)[C@@H]2CC[C@H](O2)C3=CC(=C(C(=C3)OC)OC)OC)OC
- InChI
-
InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1
- InChIKey
-
NZWPFJNQOZFEDT-ROUUACIJSA-N
|