Details of the Drug
General Information of Drug (ID: DMM5N8P)
| Drug Name |
MRX-2843
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1429882-07-4; UNC-2371A; UNII-2MT30EHI63; 2MT30EHI63; CHEMBL3326007; (1r,4r)-4-(2-((2-cyclopropylethyl)amino)-5-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol; UNC2371A; SCHEMBL14854108; SCHEMBL17175579; BDBM350861; BCP30180; BDBM50055490; MFCD28502224; ZINC299829706; CS-8117; SB17283; AS-35252; HY-101549; US9795606, B20; A16958; UNC2371;UNC-2371;UNC 2371;MRX 2843 ;MRX2843; Cyclohexanol, 4-(2-((2-cyclopropylethyl)amino)-5-(4-((4-methyl-1-piperazinyl)methyl)phenyl)-7H-pyrrolo(2,3-d)pyrimidin-7-yl)-, trans-; trans-(1R,4R)-4-(2-((2-cyclopropylethyl)amino)-5-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol; trans-4-(2-((2-Cyclopropylethyl)amino)-5-(4-((4-methyl-1-piperazinyl)methyl)phenyl)-7H-pyrrolo(2,3-d)pyrimidin-7-yl)cyclohexanol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
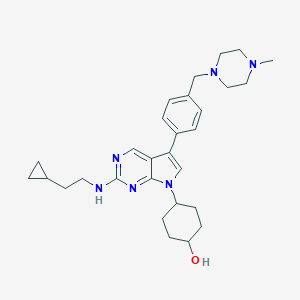 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 488.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
