Details of the Drug
General Information of Drug (ID: DMM8Z3Q)
| Drug Name |
Encequidar
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
849675-66-7; HM-30181-A; HM-30181; HM30181; UNII-K4I4I996O4; K4I4I996O4; 849675-66-7 (free base); HM 30181A; N-(2-(2-(4-(2-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)phenyl)-2H-tetrazol-5-yl)-4,5-dimethoxyphenyl)-4-oxo-4H-chromene-2-carboxamide; HM30181A; Encequidar [USAN]; HM-30181A; Encequidar (USAN/INN); HM-30181 free base; CHEMBL4594298; SCHEMBL13822558; EX-A3429A; BCP25240; MFCD25976625; WHO 10861; ZINC68014383; CS-6194; DB14070; SB18921; 4-Oxo-4H-chromene-2-carboxylic acid (2-(2-4-(2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl)-phenyl-2H-tetrazol-5-yl)-4,5-dimethoxy-phenyl)-amide; AS-35283; HY-13646; D11782; Q27281950; 4-oxo-4H-chromen-2-carboxylic acid [2-(2-{4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-phenyl}-2H-tetrazol-5-yl)-4,5-dimethoxy-phenyl]-amide; 4H-1-Benzopyran-2-carboxamide, N-[2-[2-[4-[2-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)ethyl]phenyl]-2H-tetrazol-5-yl]-4,5-dimethoxyphenyl]-4-oxo-; N-(2-(2-(4-(2-(6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-yl)ethyl)phenyl)-2H-tetrazol-5-yl)-4,5-dimethoxyphenyl)-4-oxo-4H-chromene-2-carboxamide; N-[2-[2-[4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]tetrazol-5-yl]-4,5-dimethoxyphenyl]-4-oxochromene-2-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
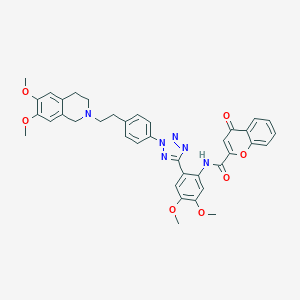 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 688.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
