Details of the Drug
General Information of Drug (ID: DMMIA38)
| Drug Name |
kassinin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
KASSININ; 63968-82-1; UNII-9BOP147TRT; 9BOP147TRT; AC1L2EKC; GTPL2088; SCHEMBL20639320; BDBM81943; NSC_45749; CAS_45749; L-alpha-Aspartyl-L-valyl-L-prolyl-L-lysyl-L-seryl-L-alpha-aspartyl-L-glutaminyl-L-phenylalanyl-L-valylglycyl-L-leucyl-L-methioninamide; LS-187667; LS-187057; L-Methioninamide, L-alpha-aspartyl-L-valyl-L-prolyl-L-lysyl-L-seryl-L-alpha-aspartyl-L-glutaminyl-L-phenylalanyl-L-valylglycyl-L-leucyl-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
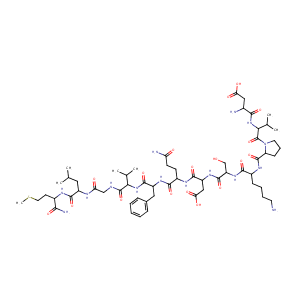 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1334.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -7.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 43 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 17 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 21 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
