Details of the Drug
General Information of Drug (ID: DMMPOEK)
| Drug Name |
alpha-ergocryptine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ergocryptine; alpha-Ergocryptine; Ergocryptine-alpha; 511-09-1; UNII-6WFB60157B; alpha-Ergokryptine; NSC 169479; CHEMBL1403281; CHEBI:10276; YDOTUXAWKBPQJW-NSLWYYNWSA-N; 12'-Hydroxy-5'alpha-isobutyl-2'-isopropylergotaman-3',6',18-trione; 6WFB60157B; EINECS 208-121-2; 12'-hydroxy-2'-(1-methylethyl)-5'alpha-(2-methylpropyl)ergotaman-3',6',18-trione; 12'-hydroxy-5'alpha-(2-methylpropyl)-3',6',18-trioxo-2'-(propan-2-yl)ergotaman; BRN 0078810; 12'-Hydroxy-2'-(1-methylethyl)-5'-alpha-(2-methylpropyl)ergotaman-3',6',18-trione
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
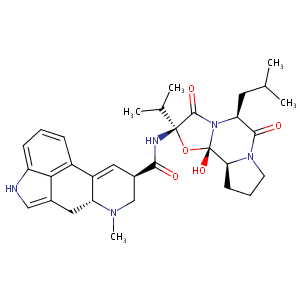 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 575.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
