Details of the Drug
General Information of Drug (ID: DMMZOYN)
| Drug Name |
ABT-348
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ILORASERTIB; ABT-348; 1227939-82-3; UNII-6L5D03D975; ABT 348; ABT348; 6L5D03D975; A-968660; Abbott-969660; Ilorasertib [USAN:INN]; Ilorasertib (USAN); Kinome_405; GTPL9914; A-968660.0; SCHEMBL3381224; CHEMBL1980297; DTXSID10153718; WPHKIQPVPYJNAX-UHFFFAOYSA-N; BCP07256; ZINC63298074; BDBM50381716; SB16853; CS-6804; DB11694; KB-74395; HY-16018; US8722890, 1; Z-3287; US8722890, 2; D10423; N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridin-3-yl}phenyl)-N'-(3-fluorophenyl)urea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
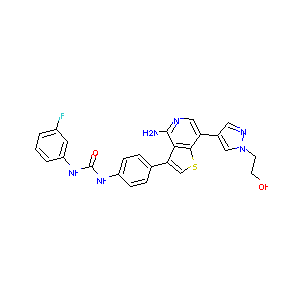 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 488.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Haematological malignancy | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2B33.Y | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
