| Drug Name |
SB 203186
|
| Synonyms |
SB 203186; SB-203186; 2-piperidin-1-ylethyl 1H-indole-3-carboxylate; Tocris-0785; NCGC00015921-01; Lopac-S-0443; Biomol-NT_000150; AC1MN68M; Lopac0_000347; GTPL255; BPBio1_000617; SCHEMBL1041116; CHEMBL1255781; ZINC14457; CHEBI:92477; BDBM85778; YGKPIROTKVQCCU-UHFFFAOYSA-N; CCG-204442; NCGC00024790-03; NCGC00015921-02; NCGC00024790-01; NCGC00024790-02; NCGC00015921-04; NCGC00015921-03; CAS_135938-17-9; (1-piperidinyl)ethyl 1h-indole 3-carboxylate; 2-(1-piperidyl)ethyl 1H-indole-3-carboxylate; 2-(1-Piperidyl)ethyl 1 H-indole-3-carboxy
|
| Drug Type |
Small molecular drug
|
| Structure |
|
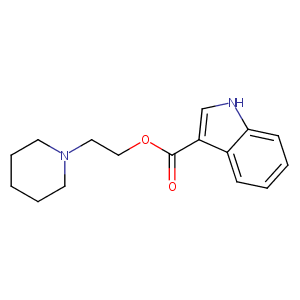 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
272.34 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.6 |
| Rotatable Bond Count (rotbonds) |
5 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
3 |
| Chemical Identifiers |
- Formula
- C16H20N2O2
- IUPAC Name
2-piperidin-1-ylethyl 1H-indole-3-carboxylate - Canonical SMILES
-
C1CCN(CC1)CCOC(=O)C2=CNC3=CC=CC=C32
- InChI
-
InChI=1S/C16H20N2O2/c19-16(20-11-10-18-8-4-1-5-9-18)14-12-17-15-7-3-2-6-13(14)15/h2-3,6-7,12,17H,1,4-5,8-11H2
- InChIKey
-
YGKPIROTKVQCCU-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 3272300
- ChEBI ID
-
- TTD ID
- D0E8KS
|
|
|
|
|
|
|
|


