Details of the Drug
General Information of Drug (ID: DMNMXCE)
| Drug Name |
4-Methyl-piperidin-(2E)-ylideneamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL6808; 2-imino-4-methylpiperidine; 2-PYRIDINAMINE, 3,4,5,6-TETRAHYDRO-4-METHYL-; 165383-71-1; 4-Methyl-piperidin-(2E)-ylideneamine; AC1MMWW1; 4-methylpiperidin-2-imine; 4-methyl-3,4,5,6-tetrahydropyridin-2-amine; 4-Methyl-2-piperidinimine; SCHEMBL4103569; CTK0A9019; DTXSID00390857; BDBM50062133; 4-Methyl-piperidin-(2Z)-ylideneamine; AKOS006348873; FT-0722141; 4-methyl-2,3,4,5-tetrahydropyridin-6-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
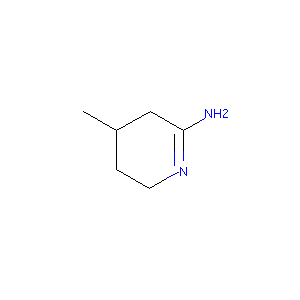 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 112.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
