Details of the Drug
General Information of Drug (ID: DMNXGZP)
| Drug Name |
6-Benzylthioinosine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
6-Benzylthioinosine; NSC26273; 6-Benzylthionebularine; MLS002702829; CHEMBL178704; 6-Benzylthiopurine ribonucleoside; 6165-03-3; NSC-26273; 6-(benzylsulfanyl)-9-pentofuranosyl-9h-purine; AC1L3TX3; AC1Q4YP0; cid_95263; ChEMBL_299712; SCHEMBL15427008; CTK5B3520; NSC 26273; BDBM50159129; SMR001566656; NCI60_002098; AI3-50272; Inosine,6-S-(phenylmethyl)-6-thio- (9CI); 9H-Purine, 6-(benzylthio)-9-.beta.-D-ribofuranosyl-; 9H-Purine, 6-(benzylthio)-9-beta-D-ribofuranosyl- (8CI); 9H-Purine, 6-((phenylmethyl)thio)-9-beta-D-ribofuranosyl-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
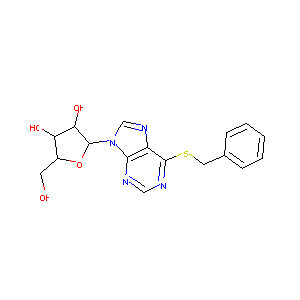 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 374.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
