Details of the Drug
General Information of Drug (ID: DMOB7P6)
| Drug Name |
2',3'-ddATP
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2',3'-Dideoxyadenosine triphosphate; ddATP; UNII-9MCI2H1EJ6; 2',3'-dideoxyadenosine-5'-triphosphate; 24027-80-3; dideoxyadenosine triphosphate; CHEMBL1383; 9MCI2H1EJ6; 2',3'-DIDEOXYADENOSINE 5'-TRIPHOSPHATE; Adenosine 5'-(tetrahydrogen triphosphate), 2',3'-dideoxy-; 2',3'-Dideoxy-ATP; ddA-TP; ATP,2',3'-dideoxy; SCHEMBL79815; GTPL1709; AC1L230G; DTXSID90178767; MolPort-044-561-436; ZINC12501706; BDBM50164644; AKOS030589611; 2'',3''-dideoxyadenosine triphosphate; DB02189; 2',3'-Dideoxyadenosine-5-triphosphate; 2',3'-Dideoxyadenosine Triphosphate (Ddatp)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
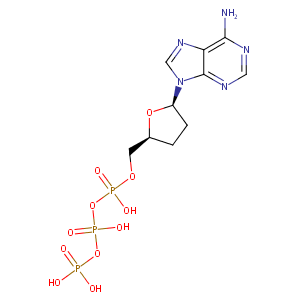 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 475.18 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
