Details of the Drug
General Information of Drug (ID: DMOJ5LM)
| Drug Name |
5,7-Dichloro-1,4-dihydro-quinoxaline-2,3-dione
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL282003; 5,7-dichloroquinoxaline-2,3-diol; 5,7-Dichloro-quinoxaline-2,3-diol; BAS 01357242; 5,7-dichloro-1,4-dihydroquinoxaline-2,3-dione; 5,7-Dichloro-1,4-dihydro-2,3-quinoxalinedione; MLS000026787; AC1LCHFV; 5,7-Dichloro-1,4-dihydro-quinoxaline-2,3-dione; Oprea1_426848; SCHEMBL7345532; SCHEMBL2639494; MolPort-002-737-915; DBSHFABBWSZJLZ-UHFFFAOYSA-N; HMS2397P18; ZINC12428297; STK782684; BDBM50008757; AKOS022421131; AKOS005619834; AKOS000543707; MCULE-5094494417; ST088493; ST042190; SMR000010423; 4029-54-3
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
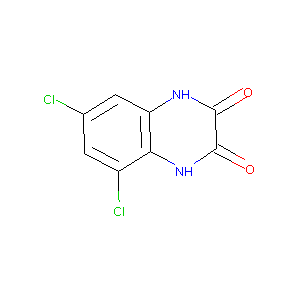 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 231.03 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
