| Synonyms |
gliotoxin; 67-99-2; Aspergillin; UNII-5L648PH06K; CCRIS 4025; Gliotoxin from Gliocladium fimbriatum; NSC 102866; BRN 0050675; AI3-62383; CHEBI:5385; CHEMBL331627; S.N. 12870; Gliotoxin, Gladiocladium fimbriatum; 5L648PH06K; 10H-3,10a-Epidithiopyrazino(1,2-a)indole-1,4-dione, 2,3,5a,6-tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-; Gliotoxins; 10H-3,10a-Epidithiopyrazino(1,2-a)indole-1,4-dione, 2,3,5a,6-hydroxy-3- (hydroxymethyl)-2-methyl-, (3R-(3-alpha,5a-beta,6-beta,10a-alpha))-
|
| Chemical Identifiers |
- Formula
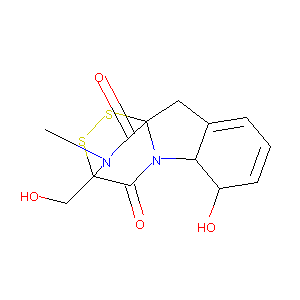
- C13H14N2O4S2
- IUPAC Name
(1R,7S,8S,11R)-7-hydroxy-11-(hydroxymethyl)-15-methyl-12,13-dithia-9,15-diazatetracyclo[9.2.2.01,9.03,8]pentadeca-3,5-diene-10,14-dione - Canonical SMILES
-
CN1C(=O)[C@]23CC4=CC=C[C@@H]([C@H]4N2C(=O)[C@]1(SS3)CO)O
- InChI
-
InChI=1S/C13H14N2O4S2/c1-14-10(18)12-5-7-3-2-4-8(17)9(7)15(12)11(19)13(14,6-16)21-20-12/h2-4,8-9,16-17H,5-6H2,1H3/t8-,9-,12+,13+/m0/s1
- InChIKey
-
FIVPIPIDMRVLAY-RBJBARPLSA-N
|