Details of the Drug
General Information of Drug (ID: DMONYS9)
| Drug Name |
MBX-102
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arhalofenate; Mbx-102; MBX 102; 24136-23-0; UNII-1P01UJR9X1; JNJ-39659100; 1P01UJR9X1; CB-102; M-102; Arhalofenate [USAN:INN]; Arhalofenate (USAN/INN); SCHEMBL3302781; CHEMBL2103824; BJBCSGQLZQGGIQ-QGZVFWFLSA-N; ZINC2012859; BDBM50093473; AKOS032945719; DB11811; CS-6812; SB16866; (-)-2-(Acetylamino)ethyl (2R)-(4-chlorophenyl)(3-(trifluoromethyl)phenoxy)acetate; CJ-31172; Benzeneacetic acid, 4-chloro-alpha-(3-(trifluoromethyl)phenoxy)-, 2-(acetylamino)ethyl ester, (-)-; HY-14831; D09579; UNII-K9TZK4MNO6 component BJBCSGQLZQGGIQ-QGZV
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
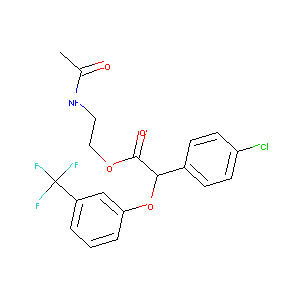 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 415.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Gout | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA25 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
