Details of the Drug
General Information of Drug (ID: DMP0ARJ)
| Drug Name |
Opaviraline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Opaviraline; GW420867X; UNII-Q3A8EU2NMU; 178040-94-3; Q3A8EU2NMU; GW-420867X; Isopropyl (2s)-2-Ethyl-7-Fluoro-3-Oxo-3,4-Dihydroquinoxaline-1(2h)-Carboxylate; isopropyl (2S)-2-ethyl-7-fluoro-3-oxo-2,4-dihydroquinoxaline-1-carboxylate; Opaviraline [INN]; HBQ; AC1L4BLF; SCHEMBL464732; Opaviraline (GW 420867); CHEMBL301370; BDBM2955; GW 867X; HBY1293; DTXSID60170415; GW867; HBY1293/GW867; ZINC3916138; HBY-1293; GW-867; AKOS027326866; DB07884; 1(2H)-Quinoxalinecarboxylic acid,2-ethyl-7-fluoro-3,4-dihydro-3-oxo-,1-methylethyl ester,(2S)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
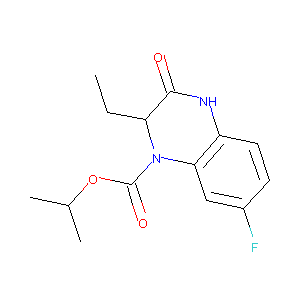 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 280.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
