Details of the Drug
General Information of Drug (ID: DMP543H)
| Drug Name |
INDOLACTUM
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(-)-Indolactam V; Indolactam V; 90365-57-4; Indolactum; UNII-8CIY9O1323; (-)-ILV; BRN 4711877; CHEMBL27266; 8CIY9O1323; (2s,5s)-5-(hydroxymethyl)-2-isopropyl-1-methyl-1,2,4,5,6,8-hexahydro-3h-[1,4]diazonino[7,6,5-cd]indol-3-one; 3H-Pyrrolo(4,3,2-gh)-1,4-benzodiazonin-3-one, 1,2,4,5,6,8-hexahydro-5-(hydroxymethyl)-1-methyl-2-(1-methylethyl)-, (2S-(2R*,5R*))-; 3H-Pyrrolo(4,3,2-gh)-1,4-benzodiazonin-3-one, 1,2,4,5,6,8-hexahydro-5-(hydroxymethyl)-1-methyl-2-(1-methylethyl)-, (2S,5S)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
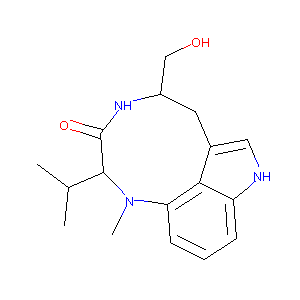 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 301.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
