Details of the Drug
General Information of Drug (ID: DMP6BSH)
| Drug Name |
Orotic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
65-86-1; 6-Carboxyuracil; Oropur; Orodin; Orotonin; Orotonsan; Oroturic; Orotyl; Whey factor; Vitamin B13; 2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid; Uracil-6-carboxylic acid; 6-Uracilcarboxylic acid; Animal galactose factor; Orotsaeure; Molkensaeure; Uracil-6-carbosaeure; 4-Pyrimidinecarboxylic acid, 1,2,3,6-tetrahydro-2,6-dioxo-; 2,6-Dihydroxypyrimidine-4-carboxylic acid; 2,6-Dihydroxy-4-pyrimidinecarboxylic acid; Orotsaeure [German]; Acidum oroticum; Acide orotique; Acido orotico; Acidum oroti
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
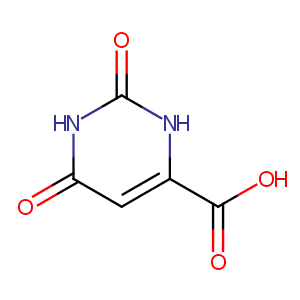 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 156.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
