Details of the Drug
General Information of Drug (ID: DMP9GFZ)
| Drug Name |
DAU-6285
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dau 6285; CHEMBL552854; (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 6-methoxy-2-oxo-3H-benzimidazole-1-carboxylate; AC1L2WHB; GTPL246; SCHEMBL2945410; CHEMBL1194556; PDSP1_000035; BDBM50007863; PDSP2_000035; PDSP1_001680; PDSP2_001663; L000897; 6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carboxylic acid 8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl ester
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
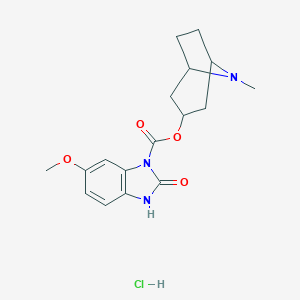 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 367.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Vomiting | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | MD90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
