Details of the Drug
General Information of Drug (ID: DMPITR9)
| Drug Name |
5-boronothiophene-2-carboxylic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
465515-31-5; 2-Carboxythiophene-5-boronic acid; 5-Carboxythiophene-2-boronic acid; 5-boronothiophene-2-carboxylic acid; 5-(dihydroxyboryl)thiophene-2-carboxylic acid; 5-(Dihydroxyboryl)-2-thiophenecarboxylic acid; C5H5BO4S; CHEMBL573906; 5-(dihydroxyboranyl)thiophene-2-carboxylic acid; AF-399/25053010; AK-26004; PubChem7851; AC1MCWDJ; ACMC-1AP1P; SCHEMBL363172; KS-00000JEI; CTK1D5505; 2-Carboxythiophene-5-boronicacid; DTXSID90381557; MolPort-000-144-982; OQGIKNPOYTVNNF-UHFFFAOYSA-N; 2-Carboxy-5-thiopheneboronic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
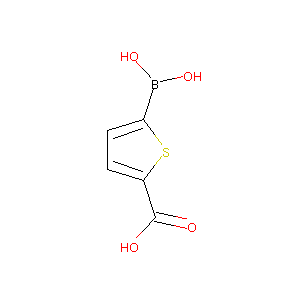 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 171.97 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
