Details of the Drug
General Information of Drug (ID: DMPOAB7)
| Drug Name |
1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3654-49-7; (1E,4E)-1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one; 1,5-Bis(4-hydroxyphenyl)penta-1,4-dien-3-one; Bis-1,5-(4-Hydroxyphenyl)-1,4-Pentadien-3-one; 1,5-Bis-(4-hydroxyphenyl)-1,4-pentadien-3-one; CHEMBL129134; EINECS 222-896-4; AC1O5NFJ; 1,5-Di(p-hydroxyphenyl)-1,4-pentadien-3-one; 1,5-BIS(4-HYDROXYPHENYL)-1,4-PENTADIEN-3-ONE; 4,4'-Dihydroxydistyrylketon; ZINC6092599; BDBM50067044; AKOS015962226; CD-1056; M064; CC-03405; AC-16155; A823289; C-35230; 654B497; 1,5-Bis-(4-hydroxy-phenyl)-penta-1,4-dien-3-one; W-106603
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
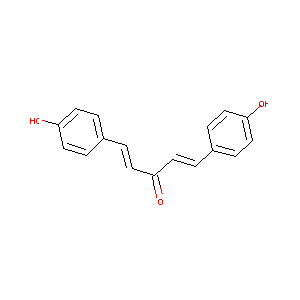 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 266.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
