Details of the Drug
General Information of Drug (ID: DMPQ8Z9)
| Drug Name |
AcAsp-Glu-Dif-Glu-Cha-Fab
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL349186; AcAsp-Glu-Dif-Glu-Cha-Fab; AC1L9WIG; BDBM50110121; Ac-L-Asp-L-Glu-3,3-Diphenyl-L-Ala-L-Glu-3-cyclohexyl-L-Ala-[(3S)-2-oxo-3-(2,2-difluoroethyl)-betaAla-]-OH; 3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylamino)-4-carboxy-butyrylamino]-3,3-diphenyl-propionylamino}-4-carboxy-butyrylamino)-3-cyclohexyl-propionylamino]-5,5-difluoro-2-oxo-pentanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
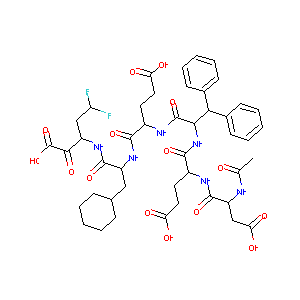 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 959 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 28 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 17 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
