Details of the Drug
General Information of Drug (ID: DMPV5D0)
| Drug Name |
L-Tryptophan-L-leucine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
H-Trp-Leu-OH; 13123-35-8; L-tryptophyl-L-leucine; CHEMBL477627; CHEBI:74871; Tryptophyl-Leucine; L-Tryptophan-L-leucine; L-Trp-L-Leu; L-Trp-L-Leu-OH; L-Leucine,L-tryptophyl-; AC1OE28P; SCHEMBL7622341; (S)-2-((S)-2-Amino-3-(1H-indol-3-yl)propanamido)-4-methylpentanoic acid; CTK4B7168; ZINC1865984; WL; BDBM50266681; AKOS022180848; AJ-32151; FT-0772989; C-48470; (2S)-2-[[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]amino]-4-methylpentanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
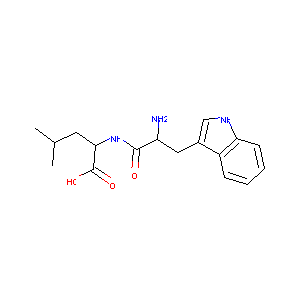 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 317.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
