Details of the Drug
General Information of Drug (ID: DMQ30X2)
| Drug Name |
HQP1351
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GZD824; 1257628-77-5; GZD-824; 3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide; UNII-KV1M7Q3CBP; KV1M7Q3CBP; 4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]benzamide; CHEMBL2316582; Benzamide, 4-methyl-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]-; HQP-1351; olverembatinib; 4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}-3-(2-{1H-pyrazolo[3,4-b]pyridin-5-yl}ethynyl)benzamide; Benzamide, 4-methyl-N-(4-((4-methyl-1-piperazinyl)methyl)-3-(trifluoromethyl)phenyl)-3-(2-(1H-pyrazolo(3,4-b)pyridin-5-yl)ethynyl)-; HQP1351 free base; HQP-1351 free base; SCHEMBL3424528; GTPL10630; EX-A829; AOB87323; BCP07502; BDBM50425780; ZINC95594040; AKOS026750647; CS-1444; SB16617; compound 10a [PMID: 23301703]; NCGC00351607-06; AK547162; AS-75170; HY-15666; D-824; FT-0700150; A16264; W-6136; J-690110; 4-Methyl-N-(4-((4-methyl-1-piperazinyl)methyl)-3-(trifluoromethyl)phenyl)-3-(2-(1H-pyrazolo(3,4-b)pyridin-5-yl)ethynyl)benzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
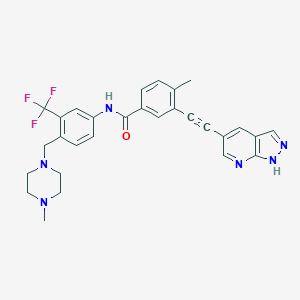 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 532.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
