Details of the Drug
General Information of Drug (ID: DMQDN46)
| Drug Name |
PLN-74809
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bexotegrast; PLN-74809; 2376257-44-0; QCV154PFT4; Pln 74809; Bexotegras; (2S)-4-(2-Methoxyethyl-(4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl)amino)-2-(quinazolin-4-ylamino)butanoic acid; (2S)-4-[2-methoxyethyl-[4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl]amino]-2-(quinazolin-4-ylamino)butanoic acid; Bexotegras [INN]; Butanoic acid, 4-((2-methoxyethyl)(4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl)amino)-2-(4-quinazolinylamino)-, (2S)-; Butanoic acid, 4-[(2-methoxyethyl)[4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl]amino]-2-(4-quinazolinylamino)-, (2S)-; bexotegrast [INN]; UNII-QCV154PFT4; CHEMBL5095257; SCHEMBL21329445; GTPL12108; US10793564, Compound 5; BDBM465216; GLXC-26826; (S)-4-((2-Methoxyethyl)(4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl)amino)-2-(quinazolin-4-ylamino)butanoic acid; PLN 74809 [WHO-DD]; PLN74809; US10793564, Compound 55; BDBM50594476; AKOS040757614; MS-29155; HY-137561; CS-0140405; (S)-4-[(2-Methoxyethyl)[4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)butyl]amino]-2-[(quinazolin-4-yl)amino]butanoic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
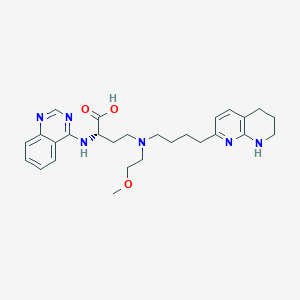 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
