Details of the Drug
General Information of Drug (ID: DMQEO7F)
| Drug Name |
9-Bromo-5,11-dimethyl-6H-pyrido[4,3-b]carbazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
9-Bromoellipticine; Ellipticine, 9-bromo-; 9-Bromo-5,11-dimethyl-6H-pyrido[4,3-b]carbazole; 18073-34-2; NSC 98927; BRN 0543507; CHEMBL194474; NSC98927; 9-Bromo-5,11-dimethyl-6H-pyrido(4,3-b)carbazole; 6H-Pyrido(4,3-b)carbazole, 9-bromo-5,11-dimethyl-; AC1L3XSF; 5-23-09-00418 (Beilstein Handbook Reference); DTXSID80171005; ZINC13284221; NSC-98927; BDBM50174409; ACM18073342; 6H-Pyrido[4, 9-bromo-5,11-dimethyl-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
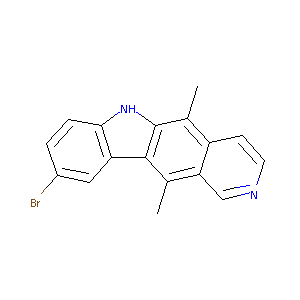 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 325.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
