Details of the Drug
General Information of Drug (ID: DMQKFM4)
| Drug Name |
AZD8871
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Navafenterol; AZD-8871; AZD8871; Navafenterol [USAN]; LAS191351; LAS-191351; U29GY32XJ4; UNII-U29GY32XJ4; Azd 8871; 1435519-06-4; WHO 11100; 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, trans-4-((3-(5-((((2R)-2-(1,2-dihydro-8-hydroxy-2-oxo-5-quinolinyl)-2-hydroxyethyl)amino)methyl)-1H-benzotriazol-1-yl)propyl)methylamino)cyclohexyl ester; NAVAFENTEROL [INN]; CHEMBL4297483; SCHEMBL16429536; SCHEMBL22766780; AZD 8871 [WHO-DD]; EX-A7799; BDBM50528210; AKOS040750679; HY-120802; CS-0079218; 2-THIOPHENEACETIC ACID, .ALPHA.-HYDROXY-.ALPHA.-2-THIENYL-, TRANS-4-((3-(5-((((2R)-2-(1,2-DIHYDRO-8-HYDROXY-2-OXO-5-QUINOLINYL)-2-HYDROXYETHYL)AMINO)METHYL)-1H-BENZOTRIAZOL-1-YL)PROPYL)METHYLAMINO)CYCLOHEXYL ESTER; TRANS-4-((3-(5-((((2R)-2-HYDROXY-2-(8-HYDROXY-2-OXO-1,2-DIHYDRO-5- QUINOLINYL)ETHYL)AMINO)METHYL)-1H-BENZOTRIAZOL-1-YL)PROPYL)(METHYL)AMINO)CYCLOHEXYL HYDROXY(DI-2-THIENYL)ACETATE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
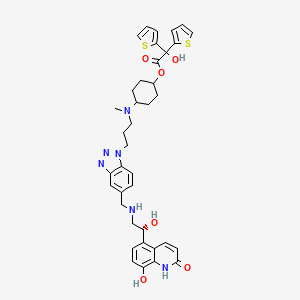 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
References
