Details of the Drug
General Information of Drug (ID: DMQPHFU)
| Drug Name |
AB-MECA
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AB-MECA; 152918-26-8; (2S,3S,4R,5R)-5-[6-[(4-aminophenyl)methylamino]purin-9-yl]-3,4-dihydroxy-N-methyloxolane-2-carboxamide; AB-MECA, solid; AC1NSJSB; [3H]AB-MECA; GTPL416; SCHEMBL16585151; CTK8E8476; BDBM21242; DTXSID70415501; LDYMCRRFCMRFKB-MOROJQBDSA-N; MolPort-003-940-100; ZINC4475069; AKOS030526575; CS-5220; NCGC00162075-03; NCGC00162075-02; NCGC00162075-01; HY-19365; RT-011146; N6-(4-Aminobenzyl)-N-methylcarboxamidoadenosine; J-008959; 5'-Methylamino-N-(4-aminobenzyl)-5'-oxo-5'-deoxyadenosine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
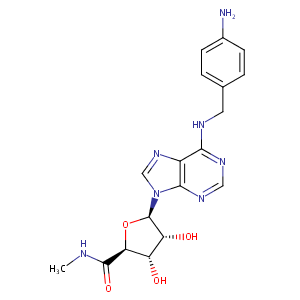 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 399.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
