Details of the Drug
General Information of Drug (ID: DMR17YI)
| Drug Name |
ISOQUINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ISOQUINE; CHEMBL147587; NINDS_000687; Spectrum_000046; Spectrum4_000147; Spectrum2_000768; Spectrum5_000808; Spectrum3_000299; AC1L1D2I; KBioSS_000426; BSPBio_001838; KBioGR_000594; DivK1c_000687; SPBio_000816; SCHEMBL5836418; KBio2_000426; KBio1_000687; KBio3_001338; KBio2_002994; CTK6E7963; KBio2_005562; ZINC36378506; BDBM50134931; IDI1_000687; NCGC00178968-01; SBI-0051280.P003; AB00053418_02; 5-(7-chloroquinolin-4-ylamino)-2-diethylaminomethylphenol; 5-[(7-chloroquinolin-4-yl)amino]-2-(diethylaminomethyl)phenol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
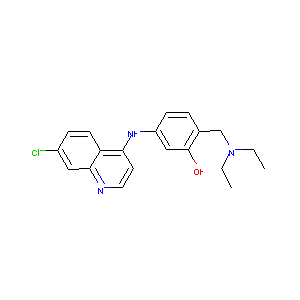 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 355.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
