Details of the Drug
General Information of Drug (ID: DMR5FD9)
| Drug Name |
SIB-1533A
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Telavancin Hydrochloride; Td 6424; 560130-42-9; TD-6424; UNII-0701472ZG0; Telavancin hydrochloride [USAN]; 0701472ZG0; Telavancin hydrochloride (USAN); Telavancin HCl; Vibativ (TN); CHEMBL3301680; CHEBI:71226; D06057; Vancomycin, N3'-(2-(decylamino)ethyl)-29-(((phosphonomethyl)amino)methyl)-, monohydrochloride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
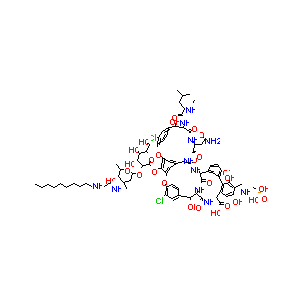 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 |
Molecular Weight | 1792.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 30 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 24 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 31 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
