Details of the Drug
General Information of Drug (ID: DMR5GSB)
| Drug Name |
Tetrahydrouridine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3,4,5,6-Tetrahydrouridine; NSC-112907; NSC 112907; CHEMBL2311128; 18771-50-1; U 23284; NSC-112907-D; BRN 0752319; 2(1H)-pyrimidinone, tetrahydro-4-hydroxy-1-beta-D-ribofuranosyl-; NSC112907; AC1L1H1G; SCHEMBL587704; 3,4,5, 6-Tetrahydrouridine; 2(1H)-Pyrimidinone, tetrahydro-4-hydroxy-1-.beta.-D-ribofuranosyl-; UCKYOOZPSJFJIZ-XVKVHKPRSA-N; MolPort-044-561-340; BDBM50421666; AKOS032953792; DB12484; LS-7719; CS-7633; HY-15345; 3,4,5,6-Tetrahydrouridine (> 80% by HPLC); J-012078
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
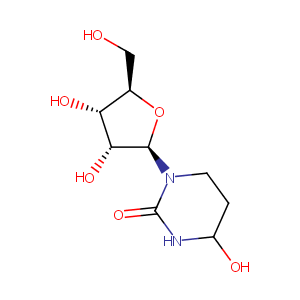 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 248.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
