Details of the Drug
General Information of Drug (ID: DMR93MK)
| Drug Name |
GRL-7234
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GRL-7234; N-[(1s,2s,4r)-2-Hydroxy-1-Isobutyl-5-({(1s)-1-[(Isopropylamino)carbonyl]-2-Methylpropyl}amino)-4-Methyl-5-Oxopentyl]-5-[methyl(Methylsulfonyl)amino]-N'-[(1r)-1-Phenylethyl]isophthalamide; 2p4j; Isophthalamide Derivative 5d; CHEMBL387771; BDBM16259; 23I; 1-N-[(1R,3S,4S)-3-hydroxy-1,6-dimethyl-1-{[(1S)-2-methyl-1-(propan-2-ylcarbamoyl)propyl]carbamoyl}heptan-4-yl]-5-(N-methylmethanesulfonamido)-3-N-[(1R)-1-phenylethyl]benzene-1,3-dicarboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
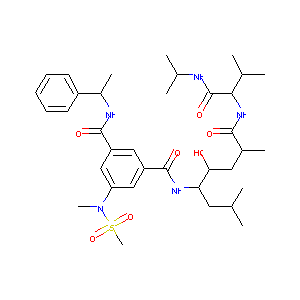 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 701.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 17 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
