Details of the Drug
General Information of Drug (ID: DMRAP4S)
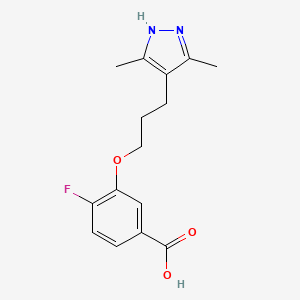
| Drug Name |
Acoramidis
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acoramidis; AG-10; Acoramidis [USAN]; AG10; 1446711-81-4; T12B44A1OE; 3-[3-(3,5-Dimethyl-1h-Pyrazol-4-Yl)propoxy]-4-Fluorobenzoic Acid; UNII-T12B44A1OE; CHEMBL3940890; 3-(3-(3,5-dimethyl-1H-pyrazol-4-yl)propoxy)-4-fluorobenzoic acid; WHO 11205; Benzoic acid, 3-(3-(3,5-dimethyl-1H-pyrazol-4-yl)propoxy)-4-fluoro-; 4hiq; ACORAMIDIS [INN]; ACORAMIDIS [WHO-DD]; SCHEMBL15816954; BDBM50197885; AKOS040759821; HY-109165; CS-0116350; E79901; Q27451739; 16V
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
