Details of the Drug
General Information of Drug (ID: DMRG0A3)
| Drug Name |
ARQ 531
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
JSFCZQSJQXFJDS-QAPCUYQASA-N; UNII-JTZ51LIXN4; JTZ51LIXN4; 2095393-15-8; ARQ-531; ARQ531; SCHEMBL18770934; SCHEMBL18756744; EX-A2727; BCP29029; HY-112215; CS-0044154; HRA; (2-Chloro-4-phenoxyphenyl)(4-(((3R,6S)-6-(hydroxymethyl)tetrahydro-2H-pyran-3-yl)amino)-7H-pyrrolo(2,3-d)pyrimidin-5-yl)methanone; 1,5-anhydro-2-{[5-(2-chloro-4-phenoxybenzene-1-carbonyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]amino}-2,3,4-trideoxy-D-erythro-hexitol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
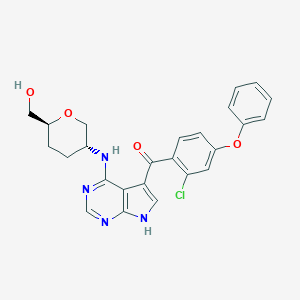 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 478.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hematologic tumour | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2B33.Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
