Details of the Drug
General Information of Drug (ID: DMROD7Q)
| Drug Name |
A-943931
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-Q598HK01AO; A-943931; Q598HK01AO; 1027330-97-7; SCHEMBL602812; CHEMBL502347; BDBM26396; MolPort-035-936-414; SDTRYHWIXVHTLM-GFCCVEGCSA-N; ZINC40977166; NCGC00370827-02; 5H-Benzo(6,7)cyclohepta(1,2-d)pyrimidin-2-amine, 4-((3R)-3-amino-1-pyrrolidinyl)-6,7-dihydro-; 2,4-diamino-5,6-disubstituted pyrimidine, 10; 6-[(3R)-3-aminopyrrolidin-1-yl]-3,5-diazatricyclo[9.4.0.0^{2,7}]pentadeca-1(15),2(7),3,5,11,13-hexaen-4-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
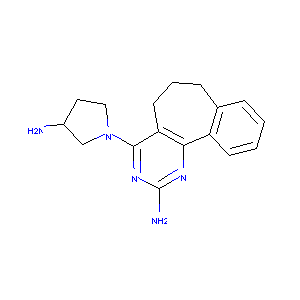 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 295.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
